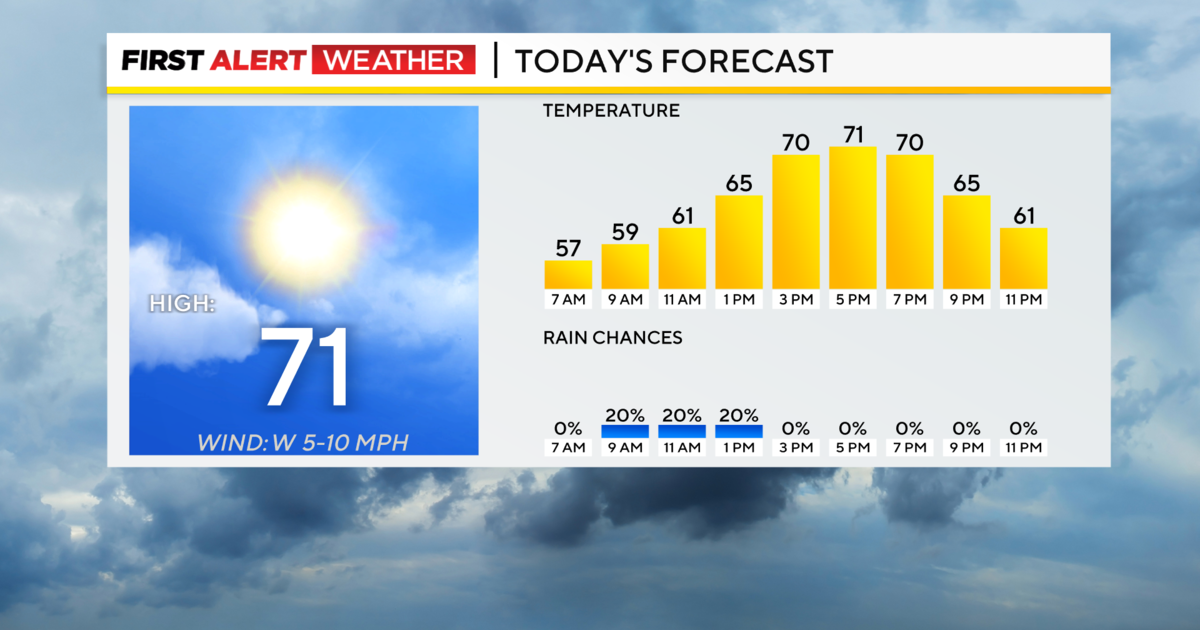
Hey Ray! 'Supercooled' Water And How It Can Freeze Right Before Your Eyes
PITTSBURGH (KDKA) -- If you ever left a bottle of water in a car or outside when temperatures were below freezing, you may have seen it turn from a liquid to ice almost instantly.
What happens to create this instant freeze is actually one of my family's favorite science experiments.
So, what is happening here? Water has a freezing point, which is usually 32 degrees.
I say "usually" because water is a neat substance that, under the right conditions can be "Supercooled."
That means, it can be cooled below its freezing point without it freezing!
Ice crystals need something in the water, an impurity, to form. Without that impurity, or nucleus, you don't get ice.
Many types of bottled water are purified, so there is nothing on which the ice crystals can form.
However, if you give it a nucleus, like a bubble that is caused when you flick the bottle or shake it up, a chain reaction of ice crystallization occurs, causing the bottle of water to freeze before your eyes.
For this experiment, you will need a bottle of purified water.
I usually do several at a time, in case they are accidentally disrupted and freeze. Put the water in the freezer.
For me, putting it in upside down works best. It is not required, just works best for me.
With a standard freezer, it takes about three hours for this water to become supercooled.
Take the bottle out of the freezer carefully. If you set off the chain reaction of ice crystallization, you will have to wait for the water to thaw, and try to super cool it again.
Now, flick the bottle, or slam it on the counter. You will see the ice crystals form throughout the water.
If you pay close attention, you will see the ice form where the nucleus was introduced, and spread from that point though the bottle.
This does not damage the water in any way. You can thaw the bottle and do this experiment over and over again.
This is just ice we're creating, so you can drink the water afterward. I did, after my experiments, so I didn't waste the water!
Now, let's try an experiment remix. What if you pour supercooled water onto a nucleus. In this case, we will use ice cubes.
They have already undergone the process of using a nucleus for the crystals to form on.
Since ice crystals are present, if you pour the supercooled water slowly on the ice cubes, you will see the ice crystals start piling up.
This experiment works much like the previous instructions.
This time, however, instead of disrupting the bottle, carefully open it, and pour it onto the ice cubes.
The same processes are happening, just outside of the bottle!
Supercooled water droplets happen in the atmosphere regularly.
Freezing drizzle and freezing fog contain super-cooled water droplets, which cause an icy glaze on things they come in contact with.
WEATHER LINKS:
Current Conditions | School Delays & Closings | Local Radar | Weather App | Photos
Stay up to date with the KDKA app, which you can download here.