Hey Ray! Boiling Water By Cooling The Air With Ice Cubes
PITTSBURGH (KDKA) -- For this experiment, we are going to boil water. It doesn't sound exciting, but we are not going to make water boil the way you traditionally do, by heating it up.
We are going to make water boil by cooling the air with ice cubes!
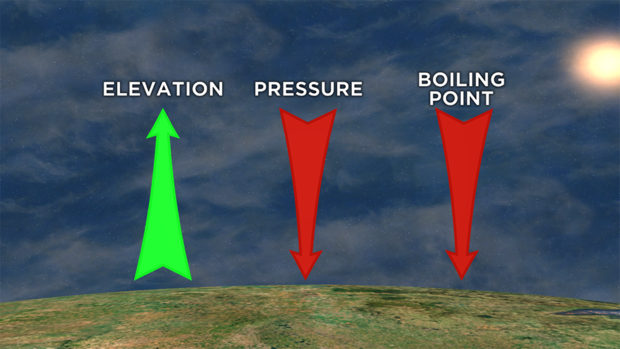
Water boils at 212°F at standard, sea level pressure. That "at standard, sea level pressure" part is important.
It tells us that the boiling point of water is dependent on pressure, so the boiling point of water can change.
You may have see this principle in action, if you cook or bake, you may have seen instructions on boxes or recipes that have different ingredients or instructions based on elevation.
This is because pressure differences cause water and heat to work differently than at sea-level.
As you increase elevation, you decrease pressure. As you decrease pressure, you decrease the boiling point of water.
So, in our experiment, we are going to trick this water into boiling at a lower temperature, by decreasing the pressure.
Much like it would act at a higher elevation, where the pressure is lower.
This experiment does involve heat, boiling water and pressure, so it should only be conducted with an adult who understands what they are doing.
You should also use safety glasses and silicone "oven mitts" or gloves while handling the flask.
READ ALL THE INSTRUCTIONS AND HAVE A GOOD UNDERSTANDING OF THE EXPERIMENT BEFORE STARTING.
YOU CAN WATCH THE VIDEO TO SEE HOW WE DID IT.
First things first, we are going to bring an Erlenmeyer flask of water to 212°F. Yes, we are going to boil it with heat. This is is going to help us drop the pressure, and we will get to the cool part, the icy part in a bit.
When water reaches its boiling point, you see bubbles form. This is not air, though. It is the water that is changing from a liquid to a gas, and that water vapor floats to the surface as bubbles. Once this happens, we will turn off the heat and add a rubber stopper. Since there is water vapor air is being lifted out of this flask around the stopper.
As the water and water vapor cool off, the water vapor begins to condense, causing the air pressure to drop, and the rubber stopper to be sucked snugly into the flask, and now, we will carefully flip it, using gloves.
REMEMBER: THIS WATER AND THE FLASK ARE STILL VERY HOT. MAKE SURE THE RUBBER STOPPER IS SECURE, AND IT IS BEST TO CONDUCT THIS PART OF THE EXPERIMENT OVER A BOWL OR POT, IN CASE THE WATER COMES OUT.
At this pressure inside the flask is lower than the pressure outside of it, so the water will act like it is at a higher elevation.
The lower pressure means the water vapor has less to overcome to break out of the liquid, making the boiling point, or the point where water changes to a vapor, lower.
We can further drop the pressure inside by adding ice cubes, condensing more of that vapor in the air above the water.
This is why we turn the flask upside down, to give a flat surface for the ice cubes to cool the air.
As the pressure drops, the boiling point of water drops even more, so our water begins to boil again, even though we are not adding any heat.
IT IS BEST NOT TO KEEP THE CORK IN THE FLASK TOO LONG. THE FLASK IS MADE OF GLASS, AND YOU ARE ACTUALLY CREATING A VACUUM BY DROPPING THE PRESSURE.
IF YOU LET IT GO TOO LONG, YOU START TO RUN THE RISK OF IMPLODING THE FLASK.