Hey Ray! Experimenting With The Hidden Heat In Rubber Bands
PITTSBURGH (KDKA) -- Sometimes, the seemingly simple things around the house may end up being much more complicated and interesting than you think.
Take, for example, rubber bands, or gum bands as they're known around here. These everyday household items have been in junk drawers and office supply closets for nearly 200 years. They were invented way back in 1845.
So, what could possibly be interesting about a rubber band?
Well, hold one under your lip and carefully stretch it. Do you feel anything weird?
Likely, it feels warmer.
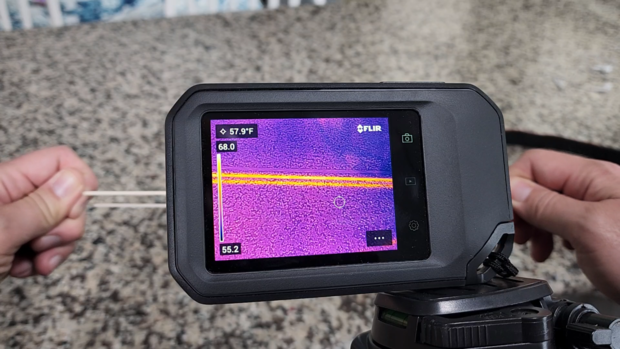
Not only does it feel warmer, though, a rubber band literally heats up when you stretch them. We can see this in action with a thermal imaging camera. As the rubber band is stretched, it heats up. When you let it go back to its resting position, it cools down.
This heating and cooling occurs because of a thing called entropy.
Entropy the measure of the amount of energy unavailable to do work.
The polymers, or chains of molecules that make up rubber bands look like an entangled pile of rubber bands when they are in their resting state. This is where they have a higher entropy...a higher amount of energy that isn't available to do work. This is how they naturally want to be.
When you stretch them, the rubber bands polymers stretch into ordered chains Because work is obtained from ordered molecular motion. Since we are doing work to stretch the rubber band and put those polymers in order, we are adding energy to the rubber band. That extra energy causes the molecules to move more rapidly, giving off heat.
Scientifically, the opposite should be true. If you let the rubber band's polymers or molecules go back to their higher state of entropy, they should absorb that heat energy and become cooler, which is something we can see with the thermal camera and feel with our skin.
You will notice we wore glasses for safety, since we were using rubber bands near our face. Always remember to protect your eyes. Also, always make sure you use a clean rubber band.